FERAHEME is the only IV iron with patient-reported outcome data in the label1
In IDA Trial 1 and the extension trial, just 1 gram of FERAHEME demonstrated:
Raised Hgb levels ≥2 g/dL at any
time from baseline to Week 51
FERAHEME
Placebo
Improvements in FACIT-Fatigue
score from baseline1
FERAHEME
Placebo
Durability of response2
of patients did not
require a second
course of treatment
over a 6-month period
IDA TRIAL 1
The majority of FERAHEME-treated patients experienced an increase of ≥2 g/dL Hgb at any time from baseline to Week 51
75.6%DIFFERENCE
(95% Cl, 71.2
to 80.0)
Fatigue-related symptoms and impacts were assessed with FACIT-Fatigue1,3*
In a 5-week study, patients treated with FERAHEME reported greater improvement from baseline in fatigue score than patients in the placebo arm1,3
4.9-pointDIFFERENCE*
(95% Cl, 3.08
to 6.71)
*Scores range from 0 to 52, with higher scores indicating less fatigue.
EXTENSION TRIAL
Duration of response in a 6-month extension trial4
For 61% of patients who were treated with FERAHEME in IDA Trial 1, mean Hgb remained stable throughout the extension study without further treatment4
Monthly Hgb levels following a single course of FERAHEME5
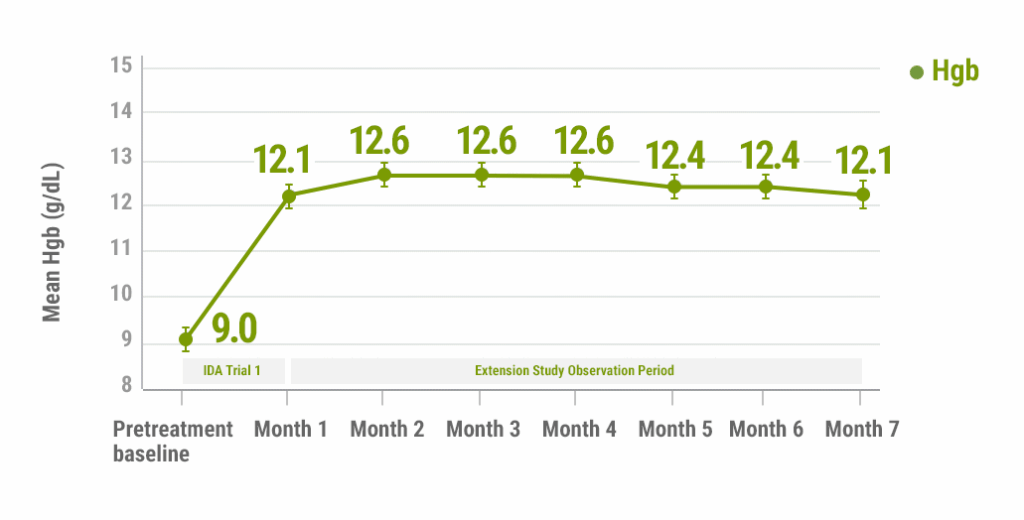
Bars represent 95% CI; Month 1 refers to both IDA Trial 1 endpoint (Week 5) and the extension study baseline.
61% of FERAHEME-treated patients
did not require a second course of treatment over the 6-month study period2
Select Important Safety Information: FERAHEME may cause clinically significant hypotension. Monitor patients for signs and symptoms of hypotension following each FERAHEME administration.
CI=confidence interval; FACIT=Functional Assessment of Chronic Illness Therapy; Hgb=hemoglobin; IDA=iron deficiency anemia; SD=standard deviation.


