FERAHEME demonstrated non-inferior efficacy in patients with and without chronic kidney disease (CKD)1
Non-CKD patients (IDA Trial 2)1,2
Comparable rates in raising Hgb levels
≥2 g/dL at any time from baseline to Week 51
FERAHEME
Venofer®
CKD patients with IDA (CKD Trial)1,3
Comparable increases in Hgb were
achieved from baseline to Week 51
FERAHEME
Venofer®
IDA TRIAL 2
FERAHEME was non-inferior to Venofer® (iron sucrose) in raising Hgb levels ≥2.0 g/dL at any time from baseline to Week 52,*
Proportion of patients with Hgb increase of ≥2.0 g/dL at any time from baseline to Week 51,2
2.6%DIFFERENCE
(95% Cl, -3.9 to 9.1)
Non-inferiority
margin: -15%
FERAHEME met the predefined criteria for non-inferiority to Venofer®.2
*Venofer® is an iron replacement product indicated for the treatment of iron deficiency anemia in patients with CKD.4
FERAHEME was non-inferior to Venofer® (iron sucrose) in mean Hgb rise from baseline2,5
Mean change in Hgb from baseline to Week 52,5†
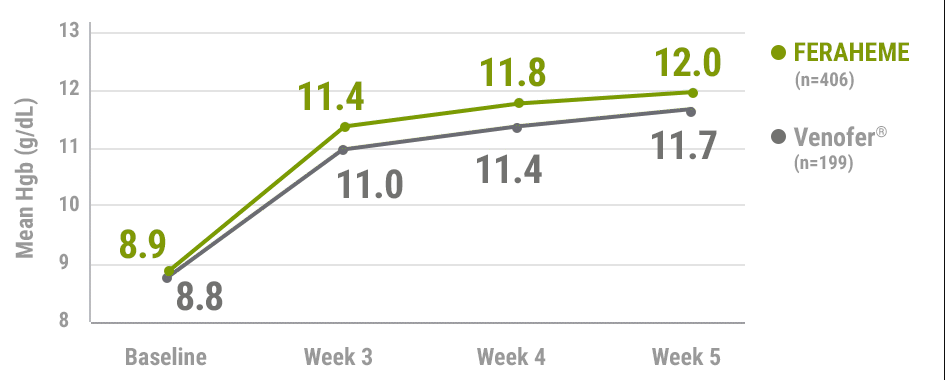
- Mean change in Hgb from baseline to Week 5 was 2.9±1.6 g/dL for FERAHEME-treated patients and 2.7±1.3 g/dL for Venofer®-treated patients (treatment difference=0.3 g/dL [95% CI, 0.06 to 0.52], non-inferiority margin: -0.5 g/dL)1,5
†n values represent the intent-to-treat population.
CI=confidence interval; Hgb=hemoglobin; IDA=iron deficiency anemia.
CKD TRIAL
In a non-inferiority study, FERAHEME demonstrated comparable efficacy with Venofer® (iron sucrose) in iron deficiency anemia patients with chronic kidney disease (CKD)3
The CKD Trial included both dialysis-dependent and non-dialysis-dependent patients1
Mean change in Hgb from baseline to Week 53
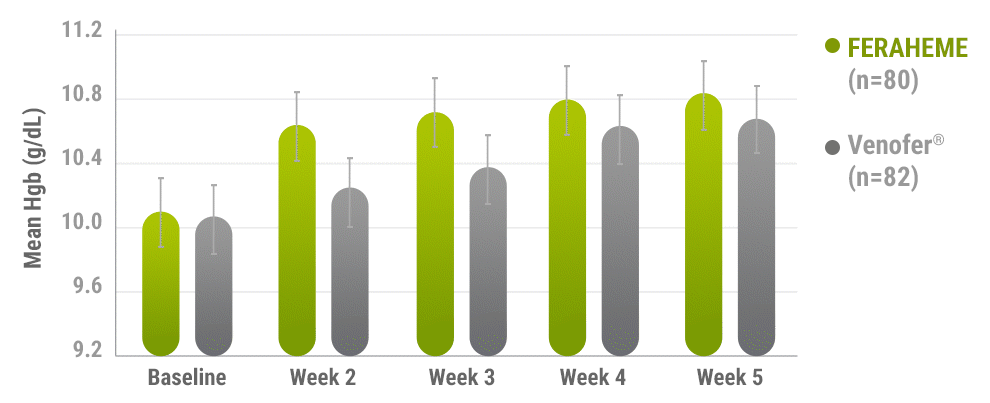
Error bars represent 95% CI.
- Comparable increases in Hgb were achieved in both treatment groups from baseline to Week 53
Primary efficacy endpoint: mean change in Hgb from baseline to Week 51,3
0.1 g/dLDIFFERENCE
(95% Cl, -0.21 to 0.41)
Non-inferiority
margin: -0.5 g/dL
96% of FERAHEME-treated patients
completed both doses of the study medication in the CKD Trial3
Select Important Safety Information: The most common adverse reactions (≥2%) are diarrhea, headache, nausea, dizziness, hypotension, constipation, and peripheral edema.
CI=confidence interval; Hgb=hemoglobin; IDA=iron deficiency anemia.


