Hypophosphatemia is associated with some IV irons1
Hypophosphatemia may go unnoticed because its clinical symptomology, such as fatigue, can overlap with iron deficiency anemia (IDA)1,2

Severe hypophosphatemia (decreased phosphorus) can lead to serious complications1:
| General weakness and fatigue1 | Headaches1 | GI symptoms such as nausea, diarrhea, and vomiting1 | Vertigo1 |
| Depression1 | Paresthesias1 | Bone pain1 | Osteomalacia3-5 |
| General weakness and fatigue1 | Headaches1 |
| GI symptoms such as nausea, diarrhea, and vomiting1 | Vertigo1 |
| Depression1 | Paresthesias1 |
| Bone pain1 | Osteomalacia3-5 |
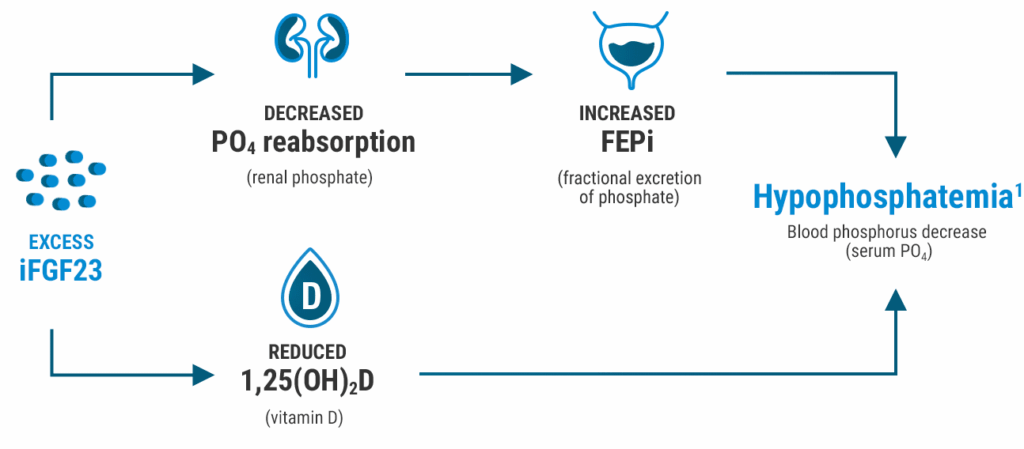
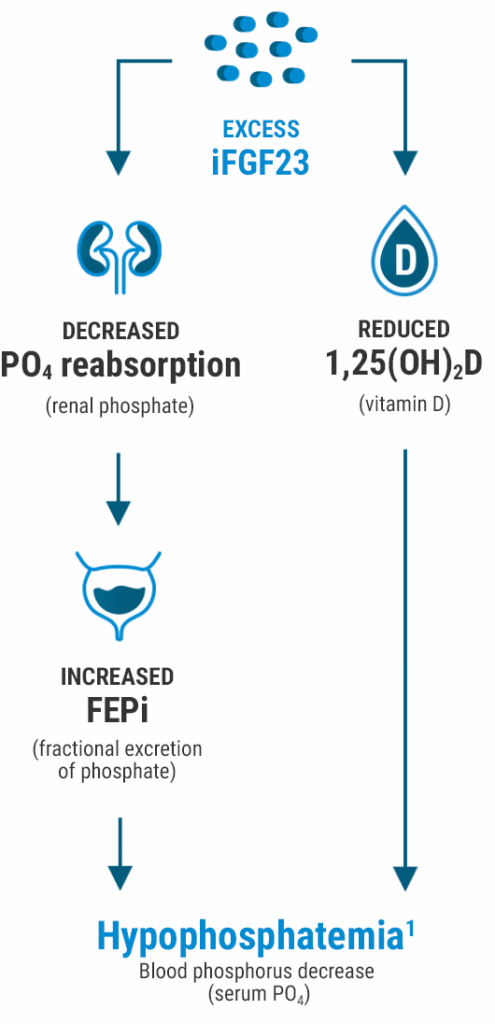
The risk of hypophosphatemia may be linked to elevated FGF23 levels caused by some IV irons6,7
- FGF23 is a hormone produced in the osteocytes that acts on the kidney to regulate phosphate homeostasis6
- Some IV irons increase levels of FGF23 (through an unknown mechanism), which can lead to hypophosphatemia1,7
FGF23=fibroblast growth factor 23; GI=gastrointestinal.
There are more than just iron levels to consider in treating your patients with iron deficiency anemia
Hypophosphatemia and its complications should be considered when prescribing IV iron1:
- Hypophosphatemia can occur despite normal pre-IV iron dose phosphate levels8
- Monitoring phosphate levels approximately 1 to 2 weeks post-infusion is recommended9,10
Choose an IV iron formulation carefully—your choice can impact patient risk of hypophosphatemia1


